
Metronidazole Tablets 200mg, 250mg, 500mg 10X10tablets/Box
Metronidazole Tablets 200mg/250mg / 500mg COMPOSITON: Metronidazole is an oral synthetic antiprotozoal and antibacterial
Send your inquiryDESCRIPTION
Basic Info
| Model NO. | 200mg, 250mg, 500mg |
| Pharmaceutical Technology | Chemical Synthesis |
| Transport Package | Carton |
| Specification | 200mg, 250mg, 500mg, 10x10tablets/box |
| Trademark | RYAN PHARMA |
| Origin | China |
Product Description
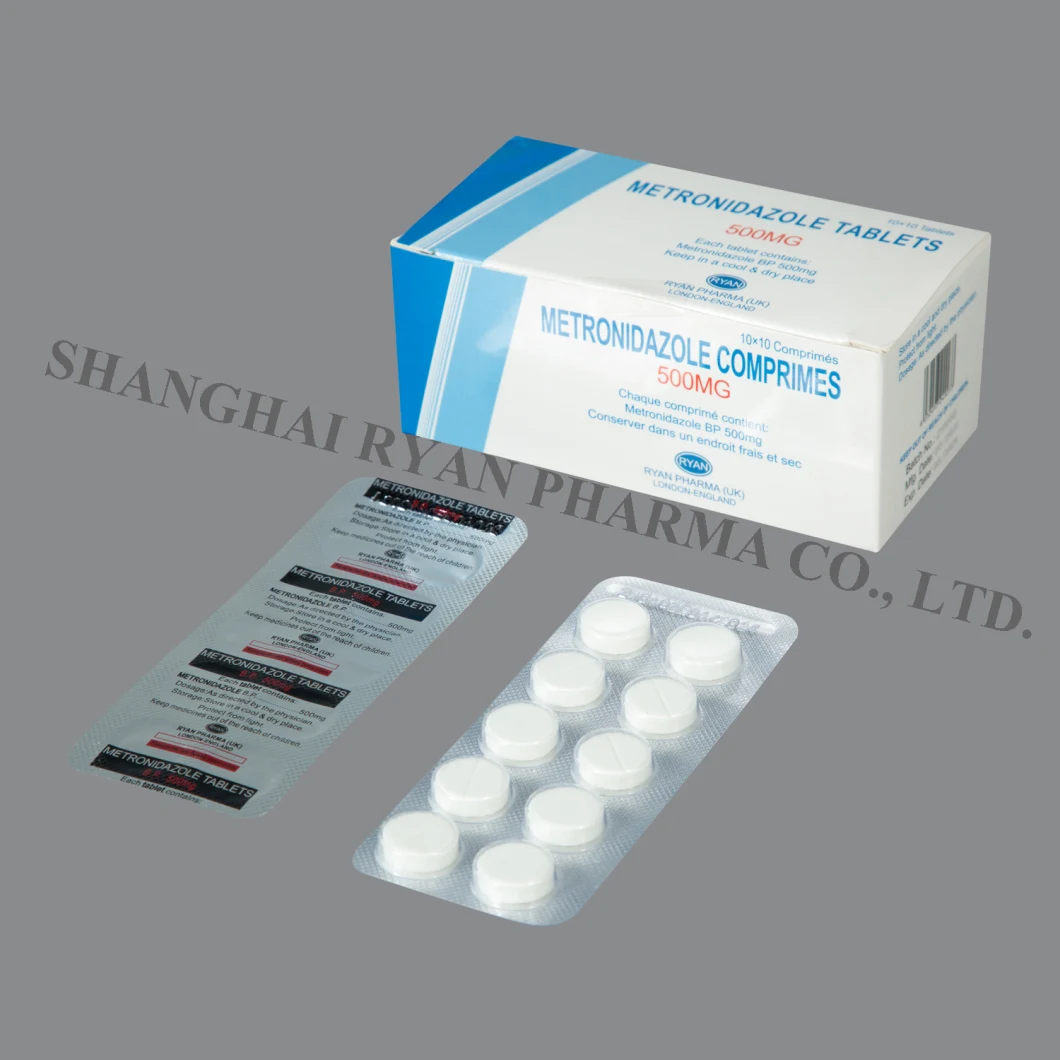
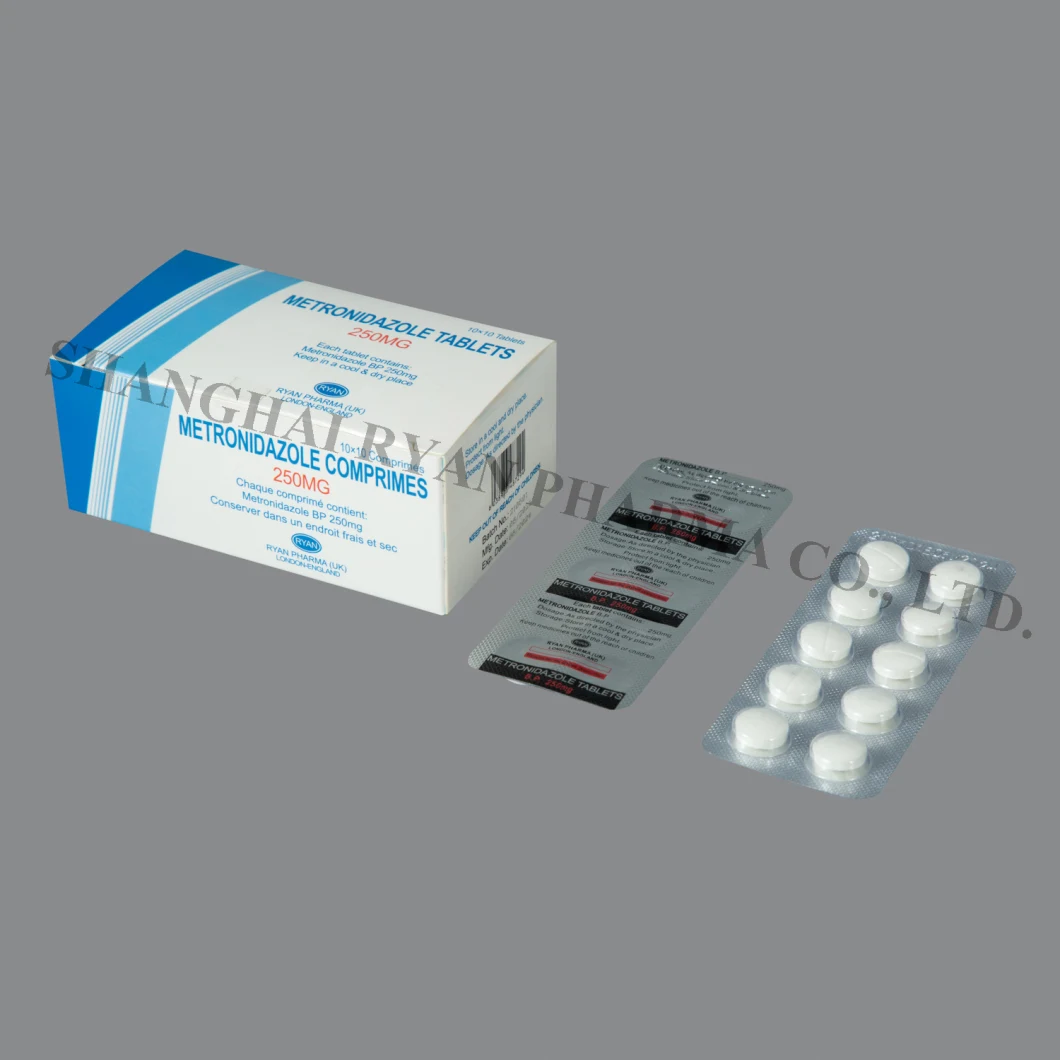
Metronidazole Tablets
200mg/250mg / 500mg
COMPOSITON:
Metronidazole is an oral synthetic antiprotozoal and antibacterial agent.
Each tablet contains metronidazole 200mg, 250mg or 500mg.
CLINICAL PHARMACOLOGY:
The major route of elimination of metronidazole and its metabolites is via the urine (60 to 80% of the dose), with fecal excretion accounting for 6 to 15% of the dose.
Metronidazole has been shown to have in vitro and clinical activity against the following organisms:
Anaerobic gram-negative bacilli, including:
Bacteroides species including the Bacteroides fragilis group (B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus) Fusobacterium species.
Anaerobic gram-positive bacilli, including:
Clostridium species and susceptible strains of Eubacterium.
Anaerobic gram-positive cocci, including:
Peptococcus species Peptostreptococcus species.
INDICATIONS:
Symptomatic Trichomoniasis: Metronidazole is indicated for the treatment of symptomatic trichomoniasis in females and males when the presence of the trichomonad has been confirmed by appropriate laboratory procedures (wet smears and/or cultures).
Asymptomatic Trichomoniasis: Metronidazole is indicated in the treatment of asymptomatic females when the organism is associated with endocervicitis, cervicitis, or cervical erosion. Since there is evidence that presence of the trichomonad can interfere with accurate assessment of abnormal cytological smears, additional smears should be performed after eradication of the parasite.
Amebiasis: Metronidazole is indicated in the treatment of acute intestinal amebiasis (amebic dysentery and amebic liver abscess).
Anaerobic Bacterial Infections: Metronidazole is indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. In a mixed aerobic and anaerobic infection, antibiotics appropriate for the treatment of the aerobic infection should be used in addition to Metronidazole.
Intra-Abdominal Infections: Including peritonitis, intra-abdominal abscess, and liver abscess, caused by Bacteroides species.
Skin and Skin Structure infections: Caused by Bacteroides species.
Gynecological Infections: Including endometritis, endomyometritis, tubo-ovarian abscess, and postsurgical vaginal cuff infection, caused by Bacteroides species including the B. fragilis group, Clostridium species, Peptococcus species, and Peptostreptococcus species.
DOSAGE AND ADMINISTRATION:
Trichomoniasis: In the Female: One-day treatment: two grams of Metronidazole given either as a single dose or in two divided doses of one gram each given in the same day. Seven-day course of treatment: 250 mg three times daily for seven consecutive days.
Amebiasis:
Adults: For acute intestinal amebiasis (acute amebic dysentery): 750 mg orally three times daily for 5 to 10 days.
For amebic liver abscess: 500 mg or 750 mg orally three times daily for 5 to 10 days.
Children: 36 to 50 mg/kg/24 hours, divided into three doses, orally for 10 days.
SIDE EFFECTS:
GI tract: The most common adverse reactions reported have been referable to the gastrointestinal tract, particularly nausea,sometimes accompanied by headache, anorexia, and occasionally vomiting; diarrhea; epigastric distress, and abdominal cramping. Constipation has been reported.
Mouth: A sharp, unpleasant metallic taste is not unusual. Furry tongue, glossitis, and stomatitis have occurred; these may be associated with a sudden overgrowth of Candida which may occur during effective therapy.
Central Nervous System: Convulsive seizures, peripheral neuropathy, dizziness, vertigo, incoordination, ataxia, confusion, irritability, depression, weakness, and insomnia.
Hypersensitivity: Urticaria, erythematous rash, flushing, nasal congestion, dryness of the mouth (or vagina or vulva), and fever.
DRUG INTERACTIONS:
Metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time.
WARNINGS:
Convulsive Seizures and Peripheral Neuropathy: The appearance of abnormal neurologic signs demands the prompt discontinuation of Metronidazole therapy. Metronidazole should be administered with caution to patients with central nervous system diseases.
CONTRAINDICATIONS:
Metronidazole is contraindicated in patients with a prior history of hypersensitivity to metronidazole or other nitroimidazole derivatives.
PRECAUTIONS:
Patients with severe hepatic disease metabolize metronidazole slowly, for such patients' doses below those usually recommended should be administered cautiously. Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with Metronidazole and requires treatment with a candicidal agent.
Pregnancy: Metronidazole crosses the placental barrier and enters the fetal circulation rapidly. This drug should be used during pregnancy only if clearly needed. Use of Metronidazole for trichomoniasis in the second and third trimesters should be restricted to those in whom local palliative treatment has been inadequate to control symptoms.
Nursing Mothers: Metronidazole is secreted in breast milk in concentrations similar to those found in plasma. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: Safety and effectiveness in children have not been established, except for the treatment of amebiasis.
SHELF LIFE:
3 Years.











